
THERE’S A VERY GOOD CHANCE YOUR INSURANCE COVERS GILENYA, AND YOUR CO-PAY COULD BE $0*
Nothing should make you compromise on
your treatment. So, here's some good news:
GILENYA is covered by most insurance plans.†

NOT AN
ACTUAL
PATIENT.
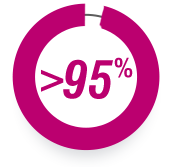
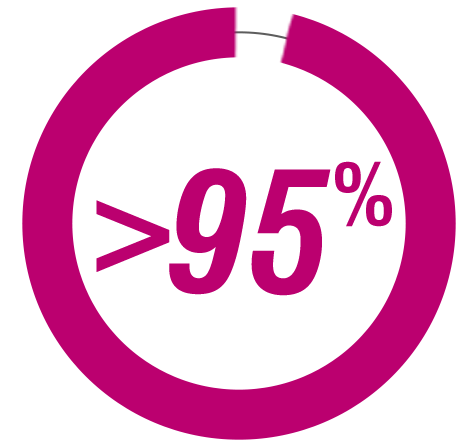
>95%
OF PEOPLE IN THE
US WITH HEALTH
INSURANCE HAVE
COVERAGE FOR
GILENYA.†

Figuring out insurance coverage is easier with the GILENYA Go Program® by your side. The Go Program can help you find out:
-
If you're eligible for a $0* co-pay
-
If you need prior authorization or approval
-
If you are eligible for any other money-saving offers
-
If you're eligible for a $0*
co-pay
-
If you need prior authorization
or approval
-
If you are eligible for any other
money-saving offers
Once you're prescribed GILENYA and your health care professional submits the Start Form, you'll automatically be enrolled in the Go Program. If you are already taking GILENYA, you can receive one-on-one ongoing support, information on your medication, and educational resources.
Enroll in the Go Program
WE OFFER 2 CO-PAY
SUPPORT PROGRAMS TO
HELP YOU GET STARTED
ON—AND STAY
ON—GILENYA:
Once you're prescribed GILENYA and your health care professional submits the Start Form, the Go Program will call you to discuss your insurance coverage, check your eligibility for our co-pay support programs, and make your treatment as affordable as possible—for many, this means a $0* co-pay.
The GILENYA Prescription Co-Pay Support Program helps commercially insured patients afford treatment by reducing the cost of your co-pay to as little as $0.* We'll even send your co-pay information directly to your pharmacy.
The GILENYA Medical Co-Pay Support Program may offer support for pretests and first-dose observation.‡
On Medicare Part D? Most people on Medicare Part D pay $0 for their GILENYA prescription each month, even though they're not eligible for co-pay support. Call the Go Program for help determining what you'll pay out of pocket.

WE OFFER 2 CO-PAY
SUPPORT PROGRAMS TO
HELP
YOU GET STARTED
ON—AND STAY
ON—GILENYA:

Once you're prescribed GILENYA and your health care professional submits the Start Form, the Go Program will call you to discuss your insurance coverage, check your eligibility for our co-pay support programs, and make your treatment as affordable as possible—for many, this means a $0* co-pay.
The GILENYA Prescription Co-Pay Support Program helps commercially insured patients afford treatment by reducing the cost of your co-pay to as little as $0.* We'll even send your co-pay information directly to your pharmacy.
The GILENYA Medical Co-Pay Support Program may offer support for pretests and first-dose observation.‡
On Medicare Part D? Most people on Medicare Part D pay $0 for their GILENYA prescription each month, even though they're not eligible for co-pay support. Call the Go Program for help determining what you'll pay out of pocket.
MAKING SURE THOSE WHO NEED OUR MEDICATIONS HAVE ACCESS TO THEM
At Novartis, we understand that you can't benefit from our medications if you can't afford them. That's why we have the following support for anyone who isn't eligible for our co-pay support programs.
- Novartis Patient Assistance NOW helps you find patient assistance programs that can help you pay for your medications. Learn more by calling 1-800-245-5356 or by visiting www.patientassistancenow.com
- The Novartis Patient Assistance Foundation, Inc. (NPAF) helps those who are experiencing financial hardship and have limited or no prescription coverage. To learn more about the NPAF, call the Go Program at 1-800-445-3692.
- Novartis Patient Assistance NOW helps you find patient assistance programs that can help you pay for your medications. Learn more by calling 1-800-245-5356 or by visiting www.patientassistancenow.com
- The Novartis Patient Assistance Foundation, Inc. (NPAF) helps those who are experiencing financial hardship and have limited or no prescription coverage. To learn more about the NPAF, call the Go Program at 1-800-445-3692.




—GILENYA COMMUNITY MEMBER


—GILENYA COMMUNITY MEMBER
GO PROGRAM is a registered trademark of Novartis AG.
*Limitations apply. Valid only for those with private insurance. The Program includes the Co-pay Card, Payment Card (if applicable), and Rebate, with a combined annual limit up to $18,000. Patient is responsible for any costs once limit is reached in a calendar year. Program not valid (i) under Medicare, Medicaid, TRICARE, VA, DoD, or any other federal or state health care program, (ii) where patient is not using insurance coverage at all, (iii) where the patient's insurance plan reimburses for the entire cost of the drug, or (iv) where product is not covered by patient's insurance. The value of this program is exclusively for the benefit of patients and is intended to be credited towards patient out-of-pocket obligations and maximums, including applicable co-payments, coinsurance, and deductibles. Program is not valid where prohibited by law. Patient may not seek reimbursement for the value received from this program from other parties, including any health insurance program or plan, flexible spending account, or health care savings account. Patient is responsible for complying with any applicable limitations and requirements of their health plan related to the use of the Program. Valid only in the United States and Puerto Rico. This Program is not health insurance. Program may not be combined with any third-party rebate, coupon, or offer. Proof of purchase may be required. Novartis reserves the right to rescind, revoke, or amend the Program and discontinue support at any time without notice.
†As of November 11, 2020. Novartis Pharmaceuticals Corporation does not endorse any particular plan. Check with your individual health plans.
‡Medical co-pay support for covered initial assessments/examinations or for first-dose observations (FDO) is provided without regard to whether the patient continues on with GILENYA therapy. People for whom GILENYA has been prescribed are required to report any benefits they receive under the GILENYA Medical Co-Pay Support Program to their insurance company. This offer is not valid for prescriptions and medical assessments for which payment may be made in whole or in part under federal or state health programs, including but not limited to Medicare or Medicaid, or for residents of RI. Valid only for those with commercial insurance. This program is subject to termination or modification at any time.








